

First Last Next Home
Iíve brought up a couple times now my idea of drawing chemistry as cartoon atoms holding hands, and I shared one example here(). Itís a lot of fun, but it gets complicated quickly and I find myself laboring over the details of how to represent the physical components and forces. Two atoms holding hands works fine for a simple covalent bond, but it breaks down when you want to represent a gradient of charge density, and that electric potential gradient is what really drives chemistry.
I tossed around several ideas for how to represent positive and negative charge but couldnít settle for a while on how to draw it. Theyíre opposites, so I thought about doing black and white, but thatís not easy to draw, and the faces are already black and white.
I thought about something like crosshatch and bubbles, but thatís really bust and the border is hard to define.
My favorite model right now is to use oil for negative charge, and cracks for positive charge. The oil looks like you dropped some oil on the ground and it splattered. And the cracks look like a mud that slowly dried. The juxtaposition between them is a little like oily and flaky/cracked skin. You need the oil to smooth it out or it starts to flake or crack. These are also fun to draw. Itís a little labor intensive, but in a digital form itís just cut and paste.
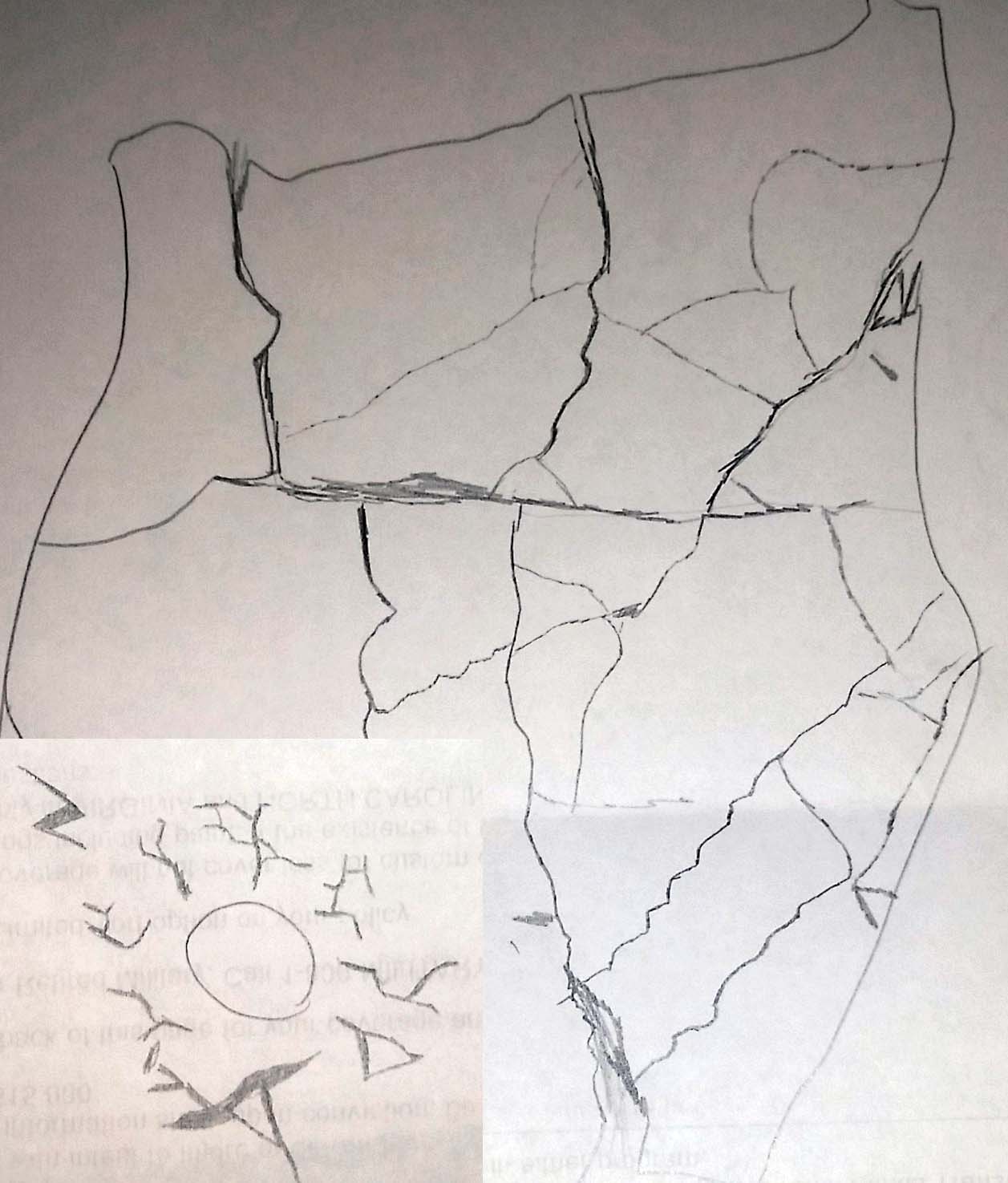
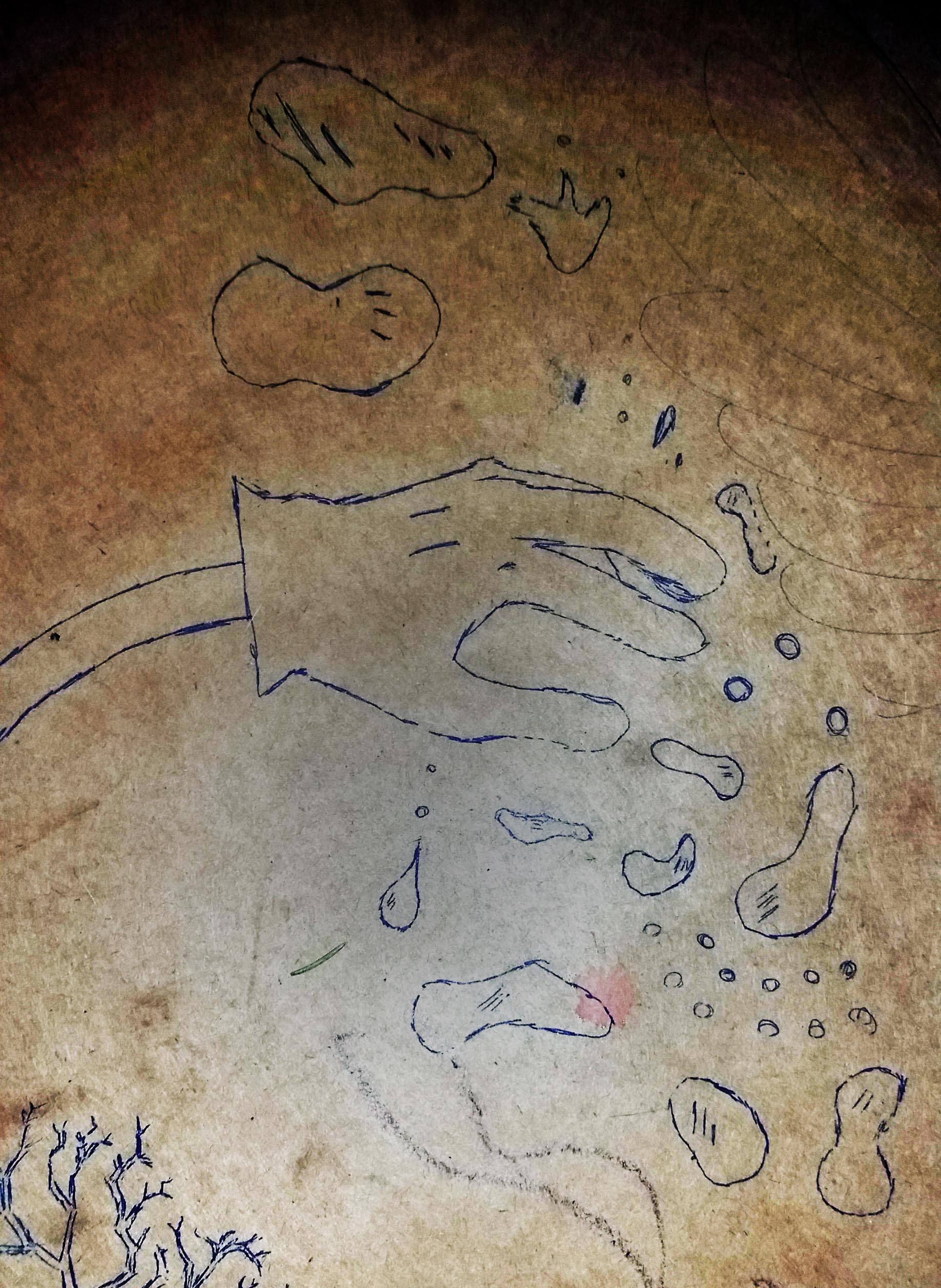
The image below is an early sketch I made looking at a google image result of some cracked mud.
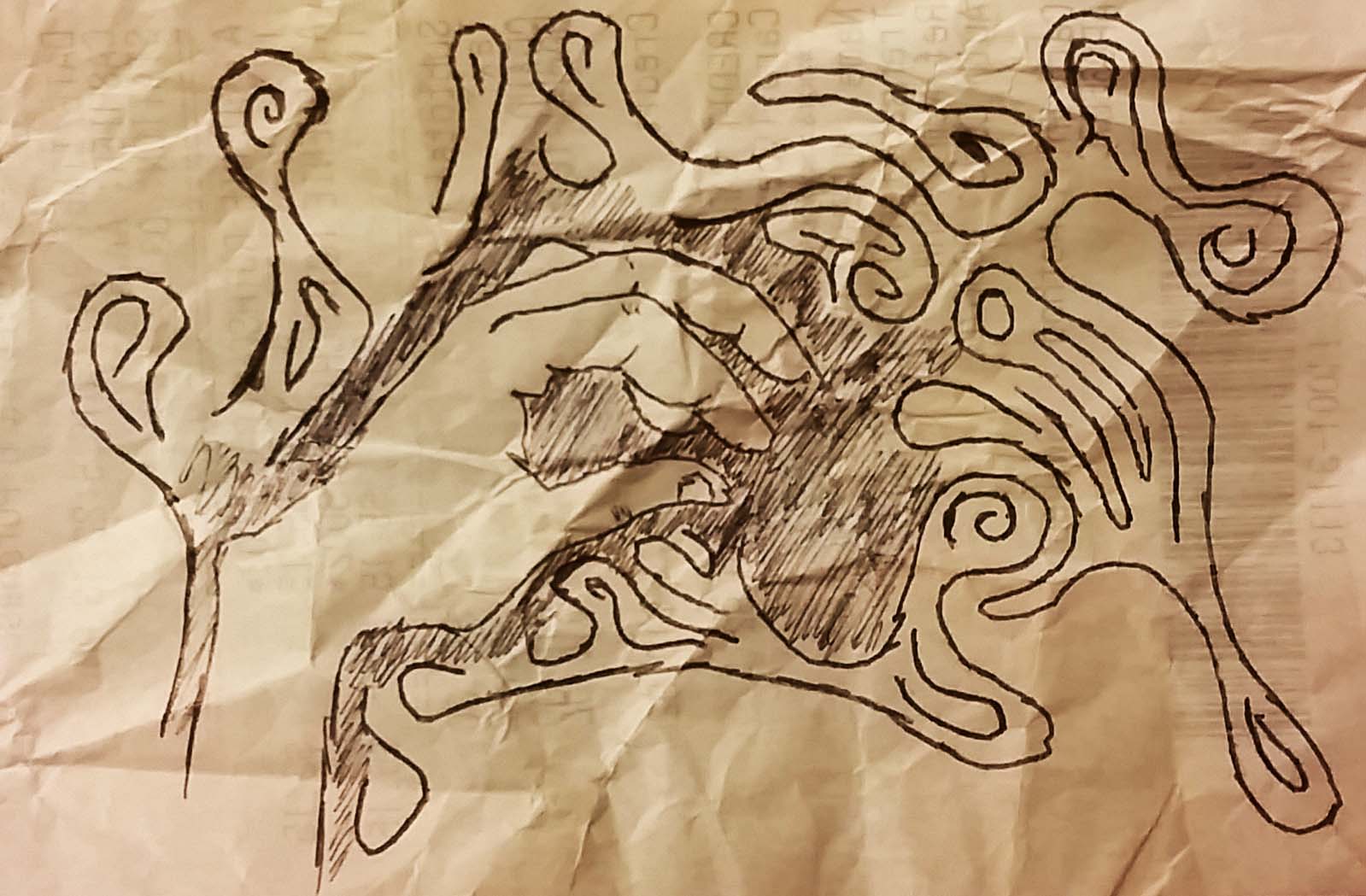
Here are a couple examples where the hand/electron is exuding negative charge in the form of oil. One of the reasons that I like this concept so much is because it reminds me of venomís suit in the spiderman comics where his alien suit is often observed oozing off into the ether. Iím linking a fan art image by NintentoGammer.
The details of how this would actually look on paper still get a little hairy. Iíd like it too look like the oil is actually filling in or smoothing out the cracks, but I havenít been able to represent it in quite the way I like yet.
This style does get pretty busy, and would only be really useful when only a handful of atoms are involved, but it only takes a handful of atoms at a time to make a reaction happen. For now, enjoy the sketches that I put together, including a rework of the helium atom from the earlier post.
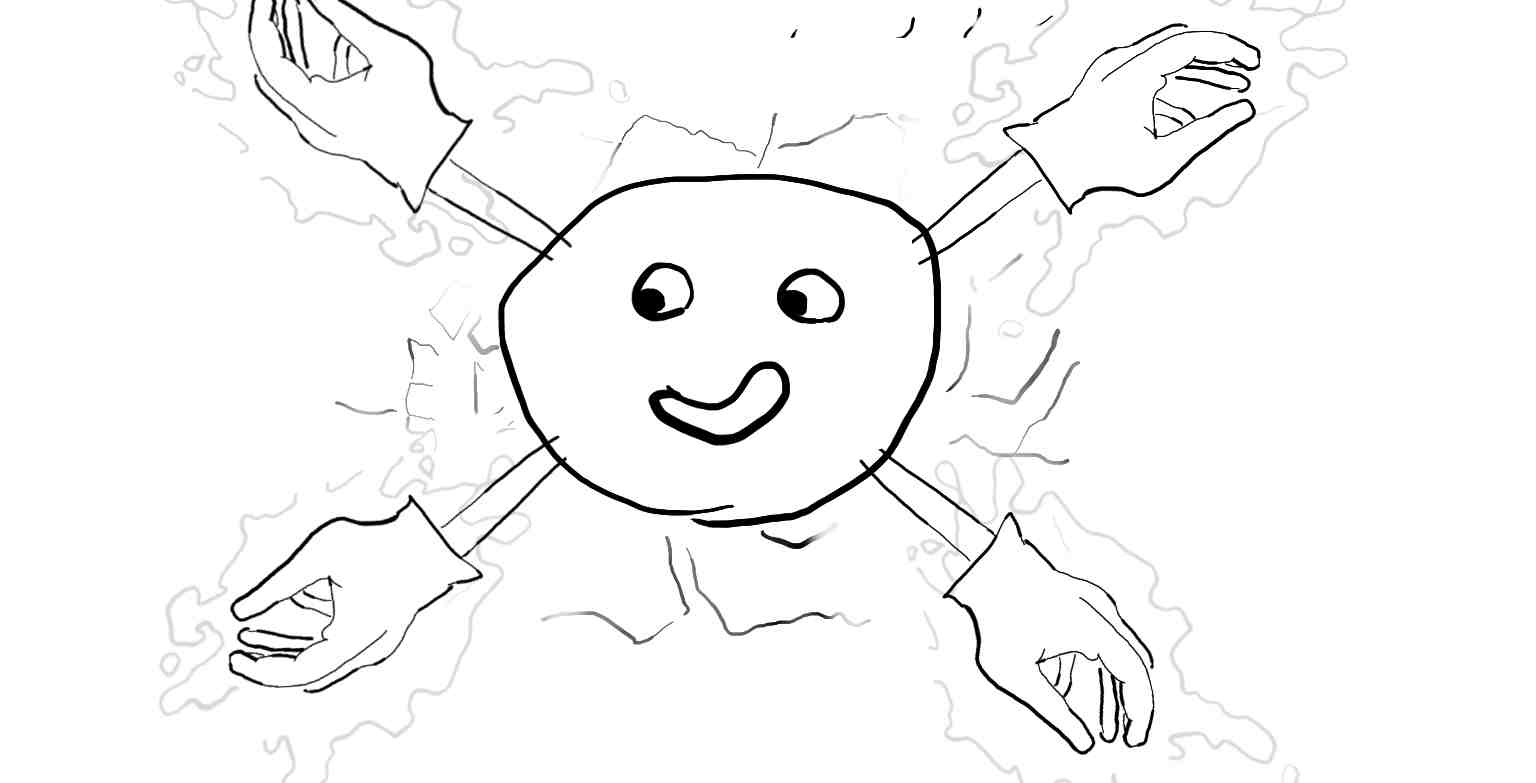
As in the earlier post the Helium atoms starts out without any electrons. This time, you can see the opsitive charge he is exuding as the cracks.

Again, the electron comes in in the form of a hand, but the hand has the field of oil around it which cancels out the positive field.

This next images represents that the electron is delocalized, and here it's sweeping across the positive charge.

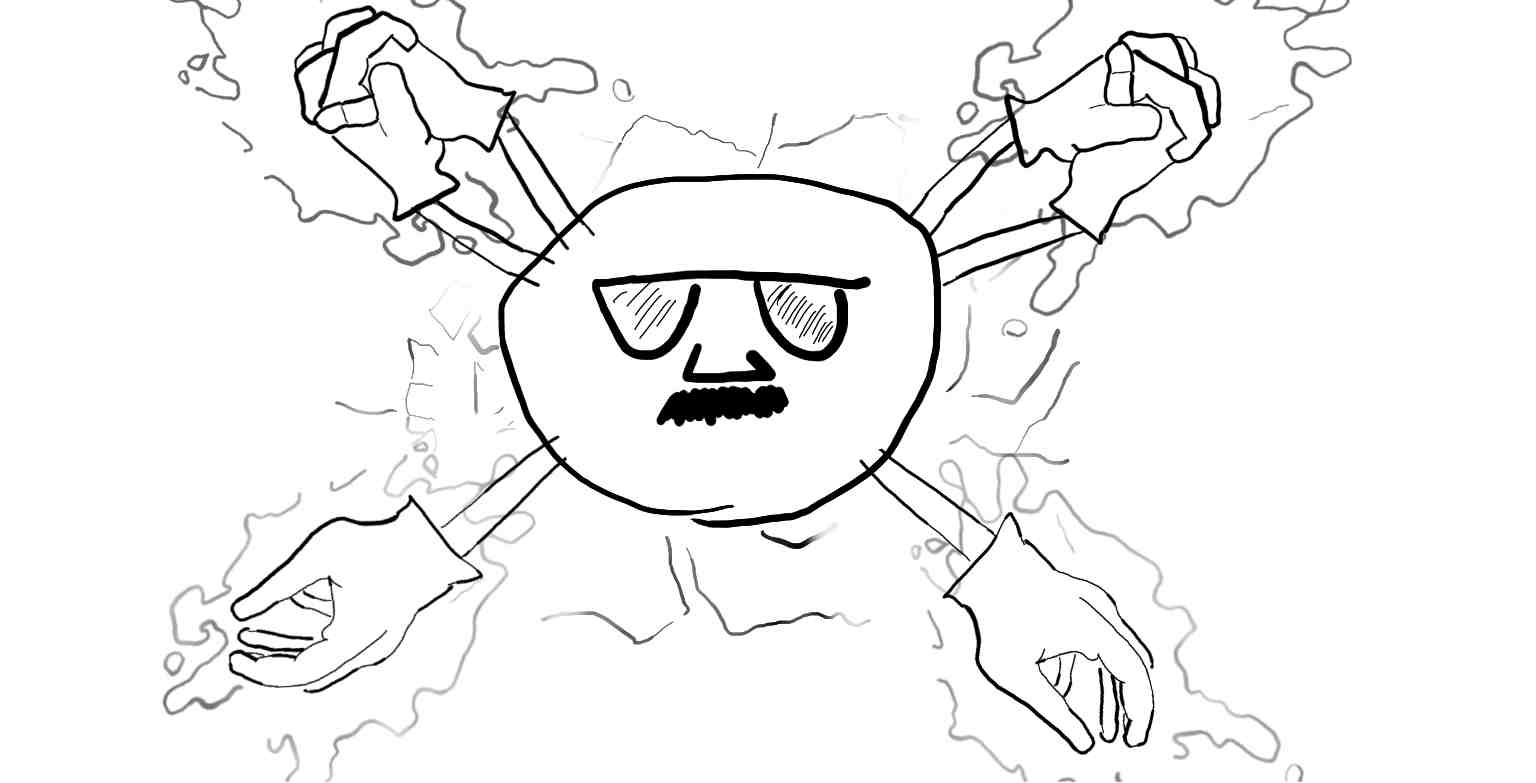
The electron will eventually cover the entire atom. In chemistry this is an s orbital. But the helium nucleus still has a charge of +2 and the cracks still prevail. In comes the next electron.
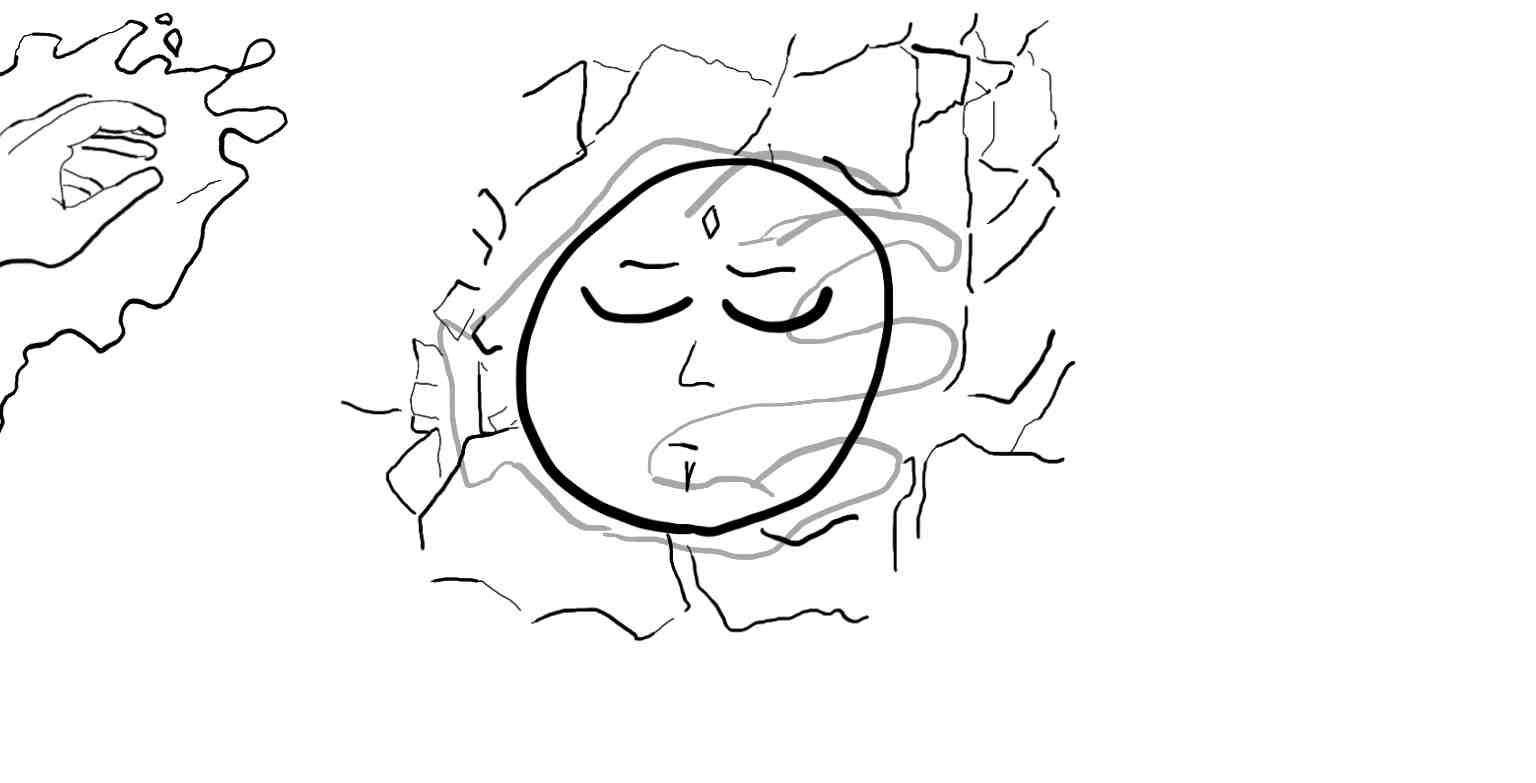
Finally the two electrons come together to form one filled s orbital and hold hands to balance out the +2 charge of the necleus and t=no charge is observed.

I'll also toss in a carbon and an oxygen atom. Their orbitals look a little more like what's shown here. You can imagine the open hand as holding hands with hydrogen atoms although they're not drawn here. I tried to show that the positive charge is concentrated at the electrons, but in between then there will be a positive charge. When multiple atoms are present, and charges start being pulled to one side, this positive exposure can be the site of nucleophilic attack. But that's a post for another day.