



First Last Next Home
In an earlier post I mentioned that I had an idea for drawing molecules as a bunch of cartoon faces holding hands. Sometimes I get these ideas while Iím driving to work without any sound on in the car. This is how I came up with ĎThe Life of Rileyí where I assign points to various activities in my life. For this cartoon chemistry and the point game Iím pretty convinced that itís a really good idea when I come up with it. I go back and forth after that, but Iím still following up with both of them a long time afterwards.
The concept is simple. Every electron is a glove on a stick, and covalent bonds are when two electrons shake hands with each other. Like any model it breaks down at some point, and I find myself patching it and adding more explanations. Itís an evolving model, and part of me would rather wait until it was finalized before sharing it. But letís face it, itís never going to be perfect. So Iíll share a little snapshot of how I explain a full valence shell for helium.
This idea is part of a larger concept in which I think that many phenomena that seem very complex could actually be explained fairly simply to a layman with the use of simple cartoons. Iím a big believer in spreading chemical education, and I would love to see more of a push to do it through cartoon concepts. So without further adieu, here is the filling of the helium 1s shell.
The first part is simple. Two atoms each have a free electron, or hand, and it wants to shake hands with another. But what about all those situations where there's an electron that doesn't want to bond with another atom. Take helium for instance. It has two electrons. Why don't those electrons want to bond with electrons from other atoms? Well the answer in chemistry is that He has a full valence shell. So those electrons are already grabbing each other, and they evenly envelop the atom. This is also how I would describe core electrons.
So you start with two electrons, and at first they look like they're ready to shake some hands.
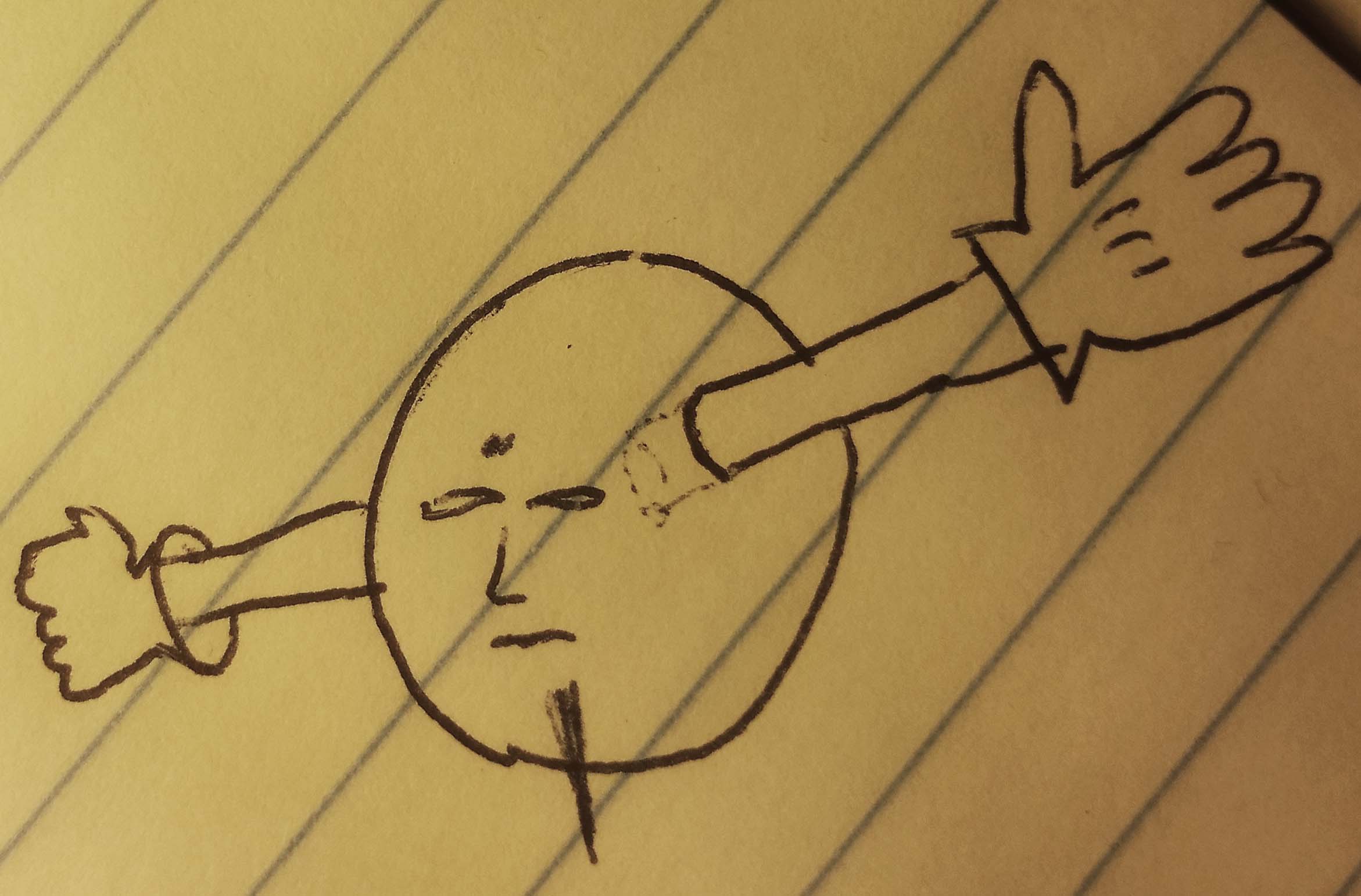
But those two electrons just ended up grabbing each other.
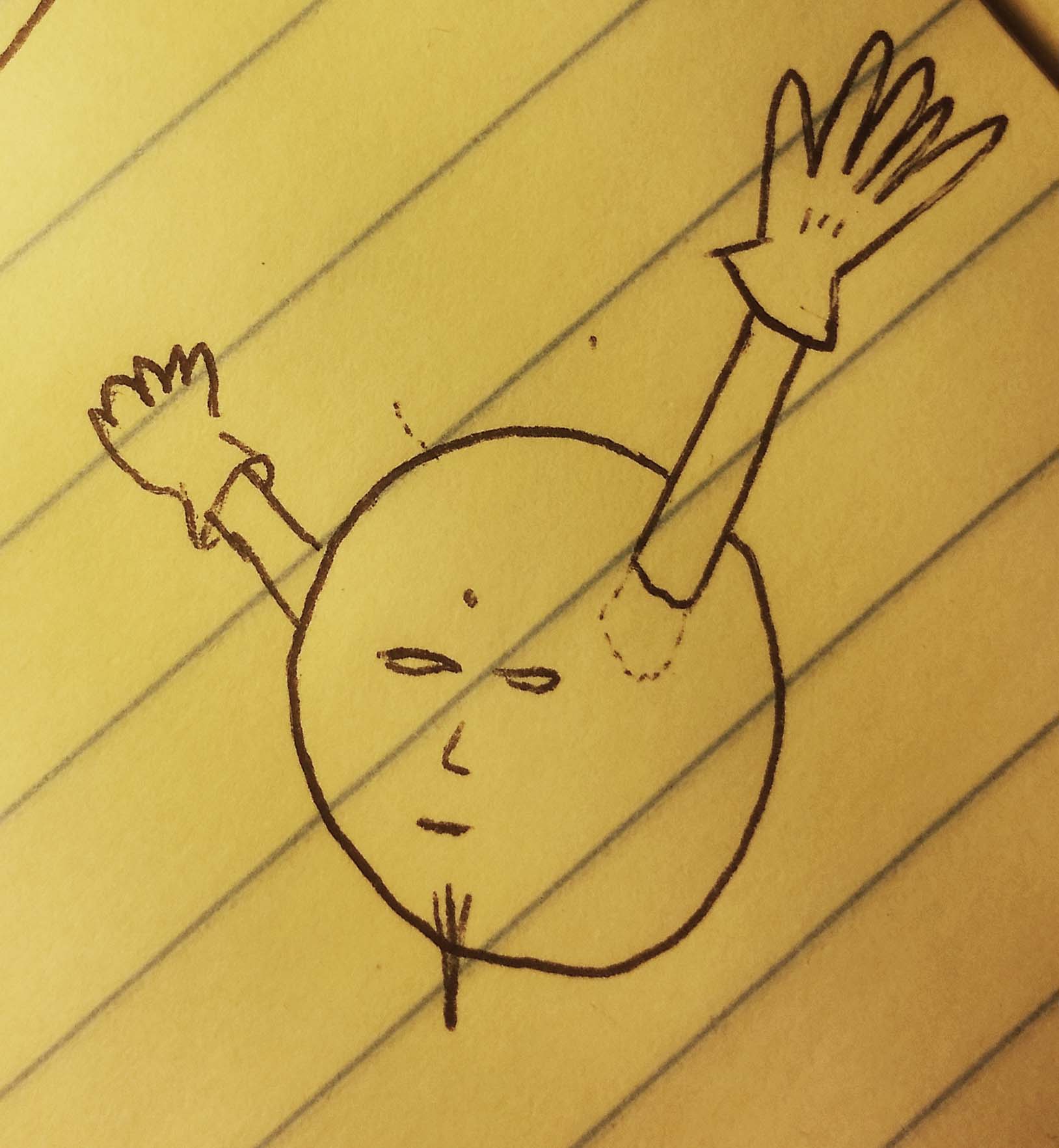
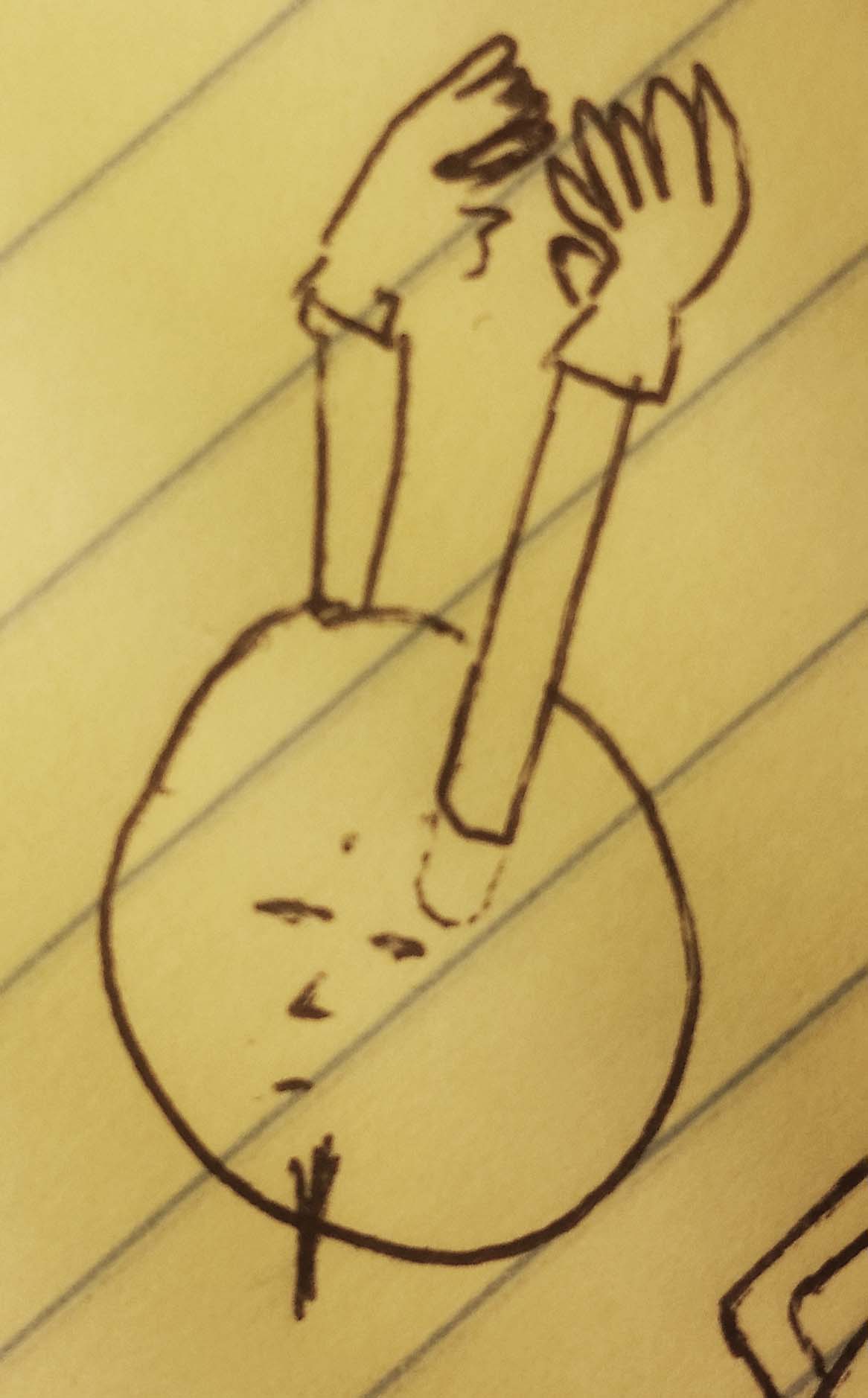
Once they grasp each other, the valence shell is filled, and they start to envelop the atom

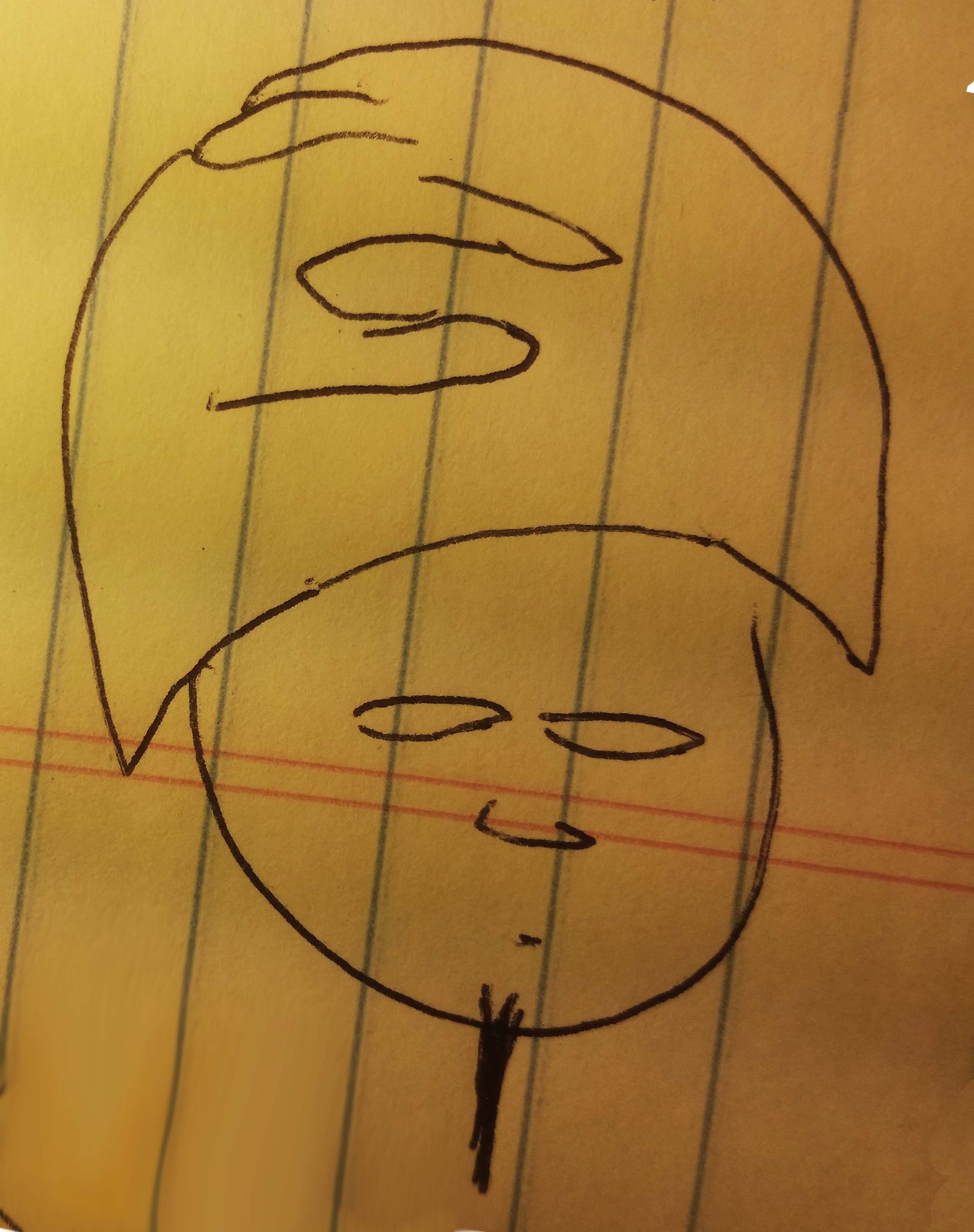

Oops, its not supposed to look lumpy. oh well. Anyway, the point is that what we have at the end is a symmetrical shell of electron/hands. When we move onto Li, with one more electron, you won't even see these electrons, they're built into the background.
And this is just one potential example for one situation. I'll explain more as I move along. So stay tuned.